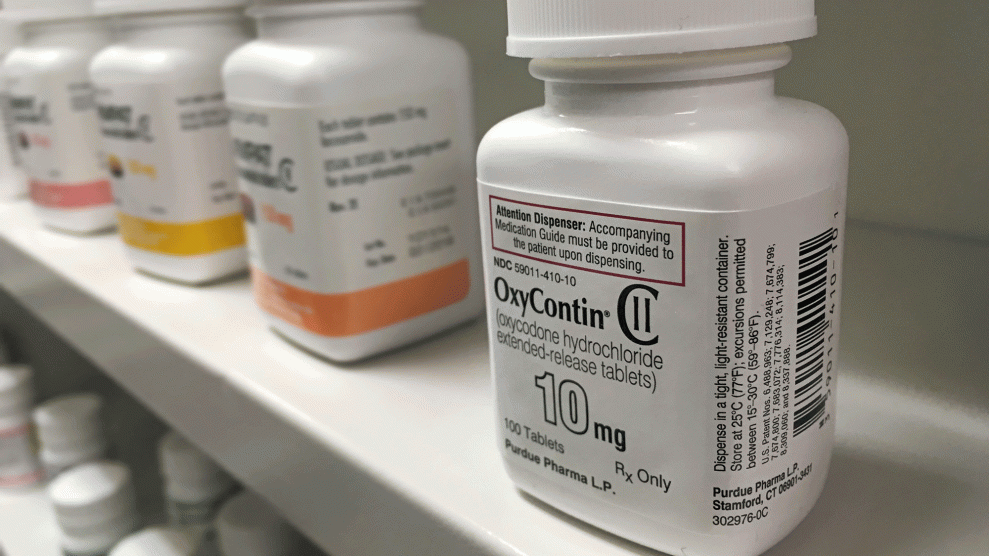
Pureradiancephoto/Getty
Opioid manufacturers funneled nearly $9 million to leading pain treatment advocacy organizations and industry groups that in turn promoted the painkillers, according to a report released Monday by Missouri Sen. Claire McCaskill (D) as part of an ongoing investigation into opioid makers.
The report examines payments between 2012 and 2017 from five pharmaceutical companies, including the makers of OxyContin and prescription fentanyl, to 14 organizations that help shape the policy and public opinion around opioids. The groups that received pharmaceutical funding—like the US Pain Foundation and the Academy of Integrative Pain Management—in turn issued guidelines minimizing the risks of opioid addiction, lobbied to change laws aimed at curbing opioid abuse, and sought to protect doctors sued for overprescribing painkillers, according to the report.
“This is part of how the game works: You have people speaking on your behalf but it’s not clear that they are,” says Keith Humphreys, a Stanford psychiatry professor and drug policy advisor under Bush and Obama. Some advocacy organizations, he said, “might as well just be a division of the companies.”
The majority of the organizations receiving money from the opioid manufacturers opposed the Centers for Disease Control and Prevention opioid prescription guidelines released in 2016, which discourage the use of the painkillers for chronic pain. The American Cancer Society Cancer Action Network and the Academy of Integrative Pain Management led a 2015 effort to protect a Tennessee law that makes it difficult to discipline doctors for overprescribing opioids, according to an investigation by the Center for Public Integrity and Associated Press. The US Pain Foundation is “actively engaged in 70 legislative bills in 20 states,” according to the organization’s annual report, and one of the foundation’s key issues is “balanced access to pain management.”
Among the five opioid manufacturers identified in the Senate report, OxyContin maker Purdue Pharma led the way, giving $4.2 million to the outside organizations over the five-year period. A Purdue statement read, “We have supported third-party organizations, including with annual dues and unrestricted grants, that are interested in helping patients receive appropriate care and share our commitment toward addressing the opioid crisis.” The company made news last week when it cut its sales team in half.
Next came Insys, the maker of the prescription fentanyl spray Subsys. Insys donated $2.5 million to the US Pain Foundation for the “Gain Against Pain” program, which provides financial assistance for medical copays of patients with acute cancer pain. (An Insys statement read, “[W]e are committed to improving the quality of patient care and bringing significant innovation to disease areas with unmet medical needs, including breakthrough cancer pain, refractory pediatric epilepsy and anaphylaxis.” A US Pain Foundation spokesman said the funding “does not influence our values.”) A previous investigation by McCaskill’s office found that Insys repeatedly misrepresented its product in order to boost sales among non-cancer patients. Company founder John Kapoor was arrested in October on federal bribery and fraud charges.
In addition to payments to advocacy organizations, pharmaceutical companies paid another $1.6 million to individuals associated with the outside organizations, including board members, staff members, and other executives. Dr. Charles Argoff, president of the American Academy of Pain Medicine Foundation, received more than $600,000 from opioid manufacturers between 2013 and 2016; over the same period, National Pain Foundation chairman Dr. Daniel Bennett received $170,000 from Insys.
McCaskill’s report notes that nearly all health advocacy organizations receive funding from pharmaceutical companies, but they aren’t by law required to report the donations. “The financial relationships between these groups and opioid manufacturers should be clear to the general public,” she said in a statement. “We passed a law ensuring the public had information on payments to doctors by pharmaceutical companies, and I can’t imagine why the same shouldn’t be done in this space.”
















